Mitochondrial plasticity and replication
Mitochondrial plasticity and replication
Basic cellular processes such as transcription, replication and DNA repair occur concomitantly in living cells. The interplay of these processes must be coordinated with cell metabolism to safeguard cellular integrity and prevent disease state. Our research group is interested in understanding the impact of micronutrients and their metabolites on these basic cellular processes and on the stability of nuclear and mitochondrial genomes.
Micronutrients such as manganese (Mn) and selenium (Se) are essential for life. However, their metabolic processing requires redox reactions that lead to the formation of reactive oxygen species (ROS), which can attack macromolecules such as lipids, proteins and DNA. Mn and Se share common features with iron (Fe) and sulfur, respectively. This is evident in the concomitant use of metal transporters in the case of Mn and Fe, and in the replacement of cysteine with selenocysteine in mammalian cells. Mn and Se may also have a direct impact on the formation of so-called iron-sulfur (Fe-S) clusters by competing with Fe or with sulfur in cluster formation. Notably, Fe-S clusters are required for the function of essential proteins involved in translation, replication or DNA repair. Intracellular Mn, Fe and Se homeostasis must be carefully regulated, since a deficiency in these nutrients reduces the activity of enzymes that protect cells from oxidative stress or DNA damage. Yet excess levels of these elements are cytotoxic and promote uncontrolled, oxidative reactions leading to disease states referred to as manganism, ferroptosis and selenosis.
KEYWORDS: Micronutrients, oxidative stress, DNA damage response, genome stability, metabolism
Research lines (led jointly by R. Wellinger and H. Gaillard):
1. Manganese homeostasis and genome stability
Enzymatic activities that require manganese (Mn) as redox-active cofactor include mitochondrial superoxide dismutase, mannosyltransferases, phosphatases or glutamate synthetase. Mn can also compete with magnesium (Mg), thereby modulating the activity of DNA polymerases or metabolic regulators like TORC1. In some instances, Mn inhibits enzymatic activities linked to retrotransposition of DNA elements or telomere elongation. Overexposure to Mn leads to oxidative stress, disrupts cellular energy metabolism, promotes aggregation and exosomal cell-to-cell transmission of α-synuclein and is a source of pathological conditions underlying neurodegeneration in humans. Accordingly, Mn neurotoxicity has been implicated in neurodegenerative diseases such as Parkinson related Manganism or Alzheimer’s disease.
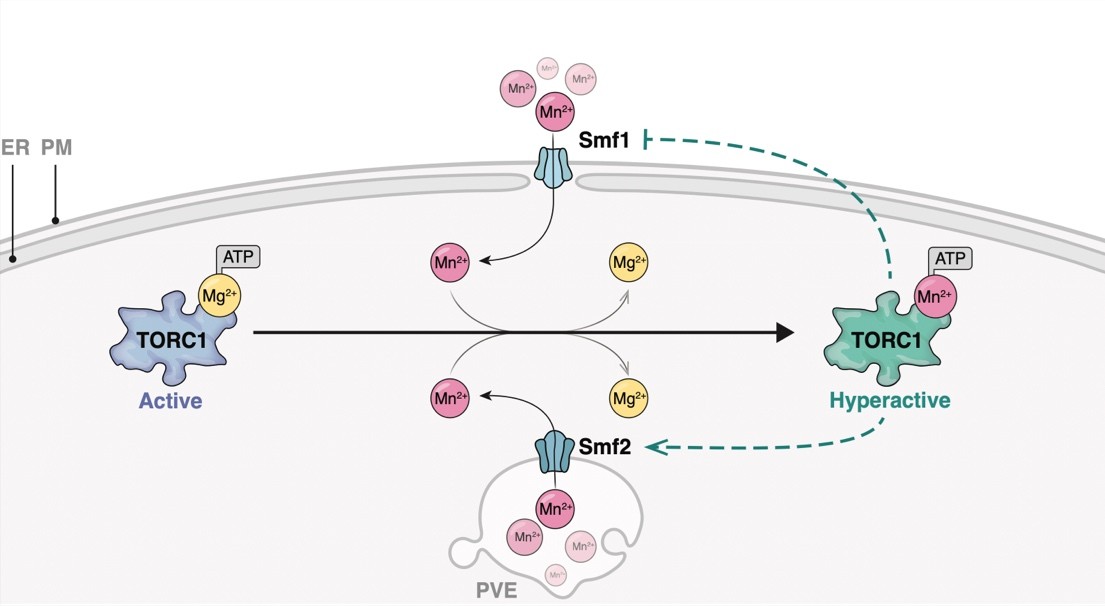
Schematical outline of yeast Mn2+-transporters and their intracellular localization. PM, plasma membrane; PVE, pre-vacuolar endosomes; ER, endoplasmic reticulum. Bsd2 is a specific adaptor protein for Rsp5-mediated Smf1 and Smf2 ubiquitination in response to Mn2+ overload. The Golgi Mn2+-transporter Gdt1 is omitted for clarity. Illustration by María Díaz de la Loza (Nicastro et al, 2022).
Much of our knowledge on Mn transport across the plasma membrane into the ER, the Golgi, endosomes, and vacuoles comes from studies in Saccharomyces cerevisiae. Typically, Mn is shuttled across membranes by transporters that belong to the natural resistance-associated macrophage protein (NRAMP) family, which are highly conserved metal transporters responsible for iron (Fe) and Mn uptake. Within cells, the P-type ATPase Pmr1 represents a key transporter that shuttles calcium (Ca) and Mn ions into the Golgi lumen. Its loss leads to increased levels of Mn in the cytoplasm due to defective Mn detoxification, and loss of Pmr1 function can be rescued by expression of the human calcium-transporting ATPase type 2C member 1 (ATP2C1, also called SPCA1). Mutations in ATP2C1 are the underlying cause of Hailey-Hailey disease (HHD). Noteworthy, several phenotypes associated with loss of Pmr1 have been shown to arise as a consequence of Mn accumulation in the cytoplasm, including telomere shortening, genome instability, and bypass of the superoxide dismutase Sod1 requirement. We investigate the factors and mechanistic causes underlying Mn induced cell damage and disease using yeast and human cells as model systems.
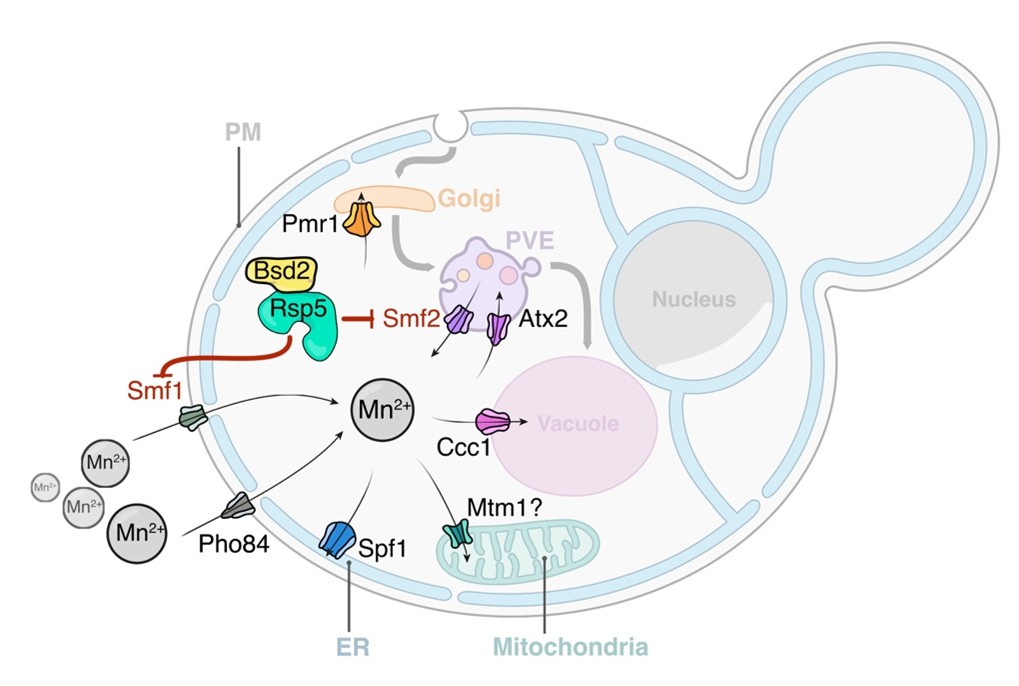
Model of Mn2+-driven TORC1 activation. The Smf1/2 NRAMP transporter-dependent increase in cytoplasmatic Mn2+ levels favors TORC1-Mn2+ binding and ATP coordination, leading to TORC1 hyperactivation. NRAMP transporters are part of a feedback control mechanism impinged by TPRC1 (dashed lines). Illustration by María Díaz de la Loza (Nicastro et al, 2022).
2. Molecular mechanisms underlying selenium induced toxicity
Selenium (Se) is an essential micronutrient that is metabolized as selenocysteine and is important for the function of so-called selenoproteins, which play an important role in the defense against oxidative stress. However, the cellular uptake and metabolism of Se include the formation of inorganic species, such as selenite and selenide, which are highly toxic to mammals. The current daily dietary reference intake for Se is as low as 55 µg, whereas the tolerable upper intake level for adults is estimated to be 400 µg. Se compounds have been shown to have preventive and therapeutic potential in the treatment of several types of cancer, including breast, ovarian and prostate cancers. However, the boundary between protective and toxic Se levels is extremely narrow, and the molecular mechanisms underlying selenite toxicity remain largely unknown. We investigate the processes involved in Se toxicity, which include oxidative stress, DNA damage, impaired mitochondrial function, protein misfolding and aggregation, and ferroptosis.
If you are interested in joining our lab as Master students, PhD students or Postdoc researchers, please contact us at ralf.wellinger@cabimer.es or helene.gaillard@cabimer.es.
Short Academic Biography for Ralf Wellinger:
Ralf Wellinger is Full Professor of Genetics at the University of Seville where he has been a faculty member since 2003. Since 2006 he is working as a Group Leader at the Andalusian Center of Molecular Biology and Regenerative Medicine (CABIMER). Ralf completed his Ph.D. at the Eidgenoessische Technische Hochschule (ETH) in Zuerich in Switzerland and his undergraduate studies at the University of Kaiserslautern in Germany. He received various fellowships including the Ramón y Cajal, Marie-Curie and EMBO programs. He speaks various languages including German, English, Spanish and French. His research interests lies in the area of genome stability ranging from yeast to human. He has collaborated actively with researchers in several other disciplines, including nano-medicine, and has founded two start-up companies.
Short Academic Biography for Hélène Gaillard:
After my Ph.D at the Swiss Federal Technology School in Zürich (ETHZ, Switzerland) working on the impact of nucleosome packaging and remodelling on the repair of UV-induced DNA lesions in vitro, I joined the team of Prof. Andrés Aguilera at the University of Seville (Spain) to study the implication of the THO complex and other RNA biogenesis factors in transcription-coupled nucleotide excision repair (TC-NER). Beside this primary research line, I started to assess the existence of an RNA-damage specific response and discovered that RNA accumulates in cytoplasmic granules upon UV irradiation. In 2011, I became Assistant Professor at the University of Seville and co-founded Suntec solar S.L., a Spin-off company of the University of Seville dedicated to the development of intelligent UV-filter to be used in cosmetics. I consolidated my expertise on the cross-talks between DNA repair, transcription, and replication in eukaryotic cells as a Senior Investigator at the CABIMER. Since January 2019, I am Associate Professor at the University of Seville and work in close collaboration with Prof. Ralf Wellinger on the impact of micronutrients on genome stability and on basic cellular processes including replication and DNA repair.
Recent Publications (5 years)
Patulin and Xestoquinol are inhibitors of DNA topoisomerase 1. E. Tumini, R.E. Wellinger, E. Herrera-Moyano, P. Navarro-Cansino, M. García-Rubio, D. Salas-Lloret, A. Losada, M.J. Muñoz-Alonso, H. Gaillard, R. Luna, A. Aguilera. PNAS (2025) 122(17):e2421167122.
Histone variant H2A.Z is needed for efficient transcription-coupled NER and genome integrity in UV challenged yeast cells. H. Gaillard*, T. Ciudad, A. Aguilera, R.E. Wellinger. PloS Genetics (2024) 20(9):e1011300. *corresponding author
Phytoplasma DNA enrichment from sugarcane white leaves for shotgun sequencing improvement. K. Lohmaneeratana, G. Gutiérrez, A. Thamchaipenet* and R.E Wellinger*. Plants MDPI (2024) 13(21):3006. *corresponding authors
Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases. I.G. de Oya, E. Jiménez-Gutiérrez, H. Gaillard, M. Molina, H. Martín, R.E. Wellinger.Int. J. Mol. Sci. (2022) 23(24):15706.
Manganese is a Physiologically Relevant TORC1 Activator in Yeast and Mammals. R. Nicastro#, H. Gaillard#, L. Zarzuela, M-P. Péli-Gulli, E. Fernández-García, M. Tomé, N. García-Rodríguez, R.V. Durán, C. De Virgilio*, R.E. Wellinger*. eLife (2022) 11:e80497. #equal contribution, *corresponding authors
A new challenge for data analytics: transposons. R.E. Wellinger and J.S. Aguilar-Ruiz. Biodata Mining (2022) 15:9.
Adaptive Response of Saccharomyces Hosts to Totiviridae L-A dsRNA Viruses Is Achieved through Intrinsically Balanced Action of Targeted Transcription Factors. B. Ravoityte, J. Luksa, R.E. Wellinger, S. Serva, E. Serviene. J. Fungi (2022) 8:381.
The human nucleoporin Tpr protects cells from RNA-mediated replication stress. M. Kosar, M. Giannattasio, D. Piccini, A. Maya-Mendoza, F. García-Benítez, J. Bartkova, S.I. Barroso, H. Gaillard, E. Martini, U. Restuccia, M.A. Ramirez-Otero, M. Garre, E. Verga, M. Andújar-Sánchez, S. Maynard, Z. Hodny, V. Costanzo, A. Kumar, A. Bachi, A. Aguilera, J. Bartek, M. Foiani. Nat. Commun. (2021) 12:3937.
The p24 complex contributes to specify arf1 for copi coat selection. S. Sabido-Bozo, A.M. Perez-Linero, J. Manzano-Lopez, S. Rodriguez-Gallardo, A. Aguilera-Romero, A. Cortes-Gomez, S. Lopez, R.E. Wellinger, M. Muñiz. Int. J. Mol. Sci. (2021) 22:423.
For a complete list of publications see
https://orcid.org/0000-0002-4421-6618
https://orcid.org/0000-0002-5740-0641








