Cellular and Molecular Neuroimmunology
The overall thrust of research in the University of Seville CMN laboratory at CABIMER is governed, to a large extent, in how to best manipulate or end inadequate immune responses as an approach to cell therapy in neurodegenerative diseases with inflammatory and/or autoimmune components.
Consequently our work has focused on understanding molecular and cellular mechanisms that regulate immune homeostasis and contribute to neuronal dysfunction and death, with particular emphasis on the role of key cell populations as microglia, dendritic cells and different T regulatory cell subsets in the development of Parkinson’s disease (PD), Multiple Sclerosis (MS), and Amyotrophic Lateral Sclerosis (ALS).
The activities at CMN laboratory merge basic disease-oriented research on primary cell cultures and cell line cultures (mouse and human), preclinical studies in mouse models of human diseases (PD, MS, ALS) and patient-driven research in clinical studies in MS and ALS.
CMN laboratory is particularly focused on sensors and transduction systems of endogenous immunomodulators (neuropeptides and specific protein complexes) for innate and adaptive immune responses in MS, PD and ALS neurodegenerative diseases.
CMN Lines of Research:
Modulation of innate and adaptive immunity by endogenous neuropeptides in neurodegeneration
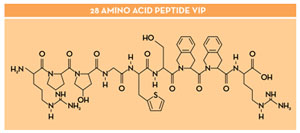 The discovery and characterization of new molecules and processes that regulate key activities on immunocompetent cells is of interest, but this is it especially when the same molecule has both neuroprotective and immunomodulatory activities. This is the case for some neuropeptides. Up to date, there are over 50 neuropeptides identified. Neuropeptides are endogenous biologically active peptides secreted by neurons or non-neural cells (in particular, we pay special attention to immunocompetent cells) that bind to specific receptors in either neurons or non-neural target cells. Thus, neuropeptides are part of the common biochemical language used by the immune and neuroendocrine systems that build a bi-directional network of major relevance in health and disease. Among them, the 28-amino acid VIP neuropeptide is a molecule (Figure 2) that has evolved from being considered a mere neuropeptide/hormone into a novel agent for modifying immune function and, possibly, as a cytokine-like molecule. VIP and the Activity-dependent Neuroprotective Protein (ADNP) are the neuropeptides under current investigation. We found that Activity-dependent Neuroprotective Protein (ADNP) has a role in the immune homeostasis of the Central Nervous System (CNS). Moreover, ADNP is known to harbor neuroprotective activities that map to the ADNP-derived sequence octapeptide called NAP (NAPVSIPQ). Cellular and molecular mechanism of action of NAP and VIP/ADNP are being investigated including its potential advantages in animal models.
The discovery and characterization of new molecules and processes that regulate key activities on immunocompetent cells is of interest, but this is it especially when the same molecule has both neuroprotective and immunomodulatory activities. This is the case for some neuropeptides. Up to date, there are over 50 neuropeptides identified. Neuropeptides are endogenous biologically active peptides secreted by neurons or non-neural cells (in particular, we pay special attention to immunocompetent cells) that bind to specific receptors in either neurons or non-neural target cells. Thus, neuropeptides are part of the common biochemical language used by the immune and neuroendocrine systems that build a bi-directional network of major relevance in health and disease. Among them, the 28-amino acid VIP neuropeptide is a molecule (Figure 2) that has evolved from being considered a mere neuropeptide/hormone into a novel agent for modifying immune function and, possibly, as a cytokine-like molecule. VIP and the Activity-dependent Neuroprotective Protein (ADNP) are the neuropeptides under current investigation. We found that Activity-dependent Neuroprotective Protein (ADNP) has a role in the immune homeostasis of the Central Nervous System (CNS). Moreover, ADNP is known to harbor neuroprotective activities that map to the ADNP-derived sequence octapeptide called NAP (NAPVSIPQ). Cellular and molecular mechanism of action of NAP and VIP/ADNP are being investigated including its potential advantages in animal models.
Nanoparticles for diagnostics and for controlled and targeted drug delivery: improving the drugability of neuropeptides
Neuropeptides are potentially valuable tools for clinical applications as they offer many distinct advantages over other bioactive molecules like proteins and monoclonal antibodies due to their reduced side effects and simple chemical modifications. Despite such advantages, the difficulty with neuropeptides often relies on their poor metabolic stability and reduced biological activity intervals.
At CMN lab an active line of research is aimed at developing nanostructured organic and inorganic systems either for the appropriate delivery of VIP or for VIP-targeting. These technologies stands as an alternative starting point for chemical manipulations of the neuropeptides in order to improve potency, selectivity, or pharmacokinetic parameters. This could facilitate the effective translation of preclinical studies related to VIP to clinical realities, most of which are commonplace for other neuropeptides and endogenous regulators of the immune system within the central nervous system.
We have the expertise to functionalized and characterize peptide-engineered nanoparticles by DLS, AFM, TEM. Besides focus on chemical synthesis of nanoparticles (NPs), we are particularly interested in the effects of the interaction of NPs with the immune system. Not only in terms of toxicity, but in terms of biocompatibility and functional status.
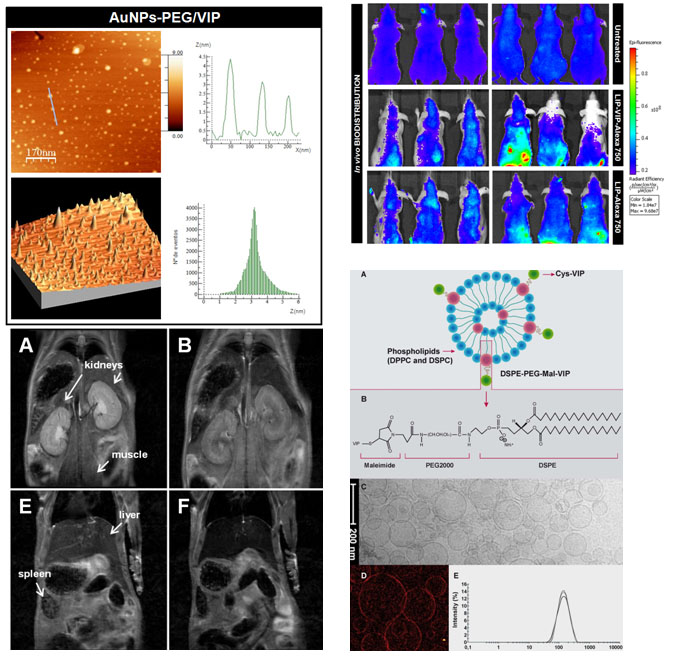
Figure 3. Several NPs are being investigated at CMN Lab for diagnostics and controlled and targeted drug delivery based on neuropeptides and other endogenous immune-modulators (CMN Lab).
Role of immune-mediated mechanisms in Amyotrophic Lateral Sclerosis (ALS)
Even though the progressive death of motor neurons is ultimately responsible for the development of ALS, in the last few years it has become accepted that multiple mechanisms involving other cell types, notably microglia activation, T cell infiltration to the CNS, and a dysfunctional adaptive immunity, have a key participation in the pathophysiology of disease. This is our activity area related to basic research in ALS. In this sense, at CMN Lab, we explore the interface between the host/innate immune systems and self-proteins with distinctive behaviour. We work with SOD1 and TDP-43 proteins (overexpressed as inclusion bodies or secreted by lentiviral transduction). Besides these mechanistic insights, we are merging molecular imaging and “omics” approaches in ALS research, in both preclinical and clinical settings. High resolution metabolomics, molecular imaging MRI and CT-PET technologies are currently extensively used in our laboratory.
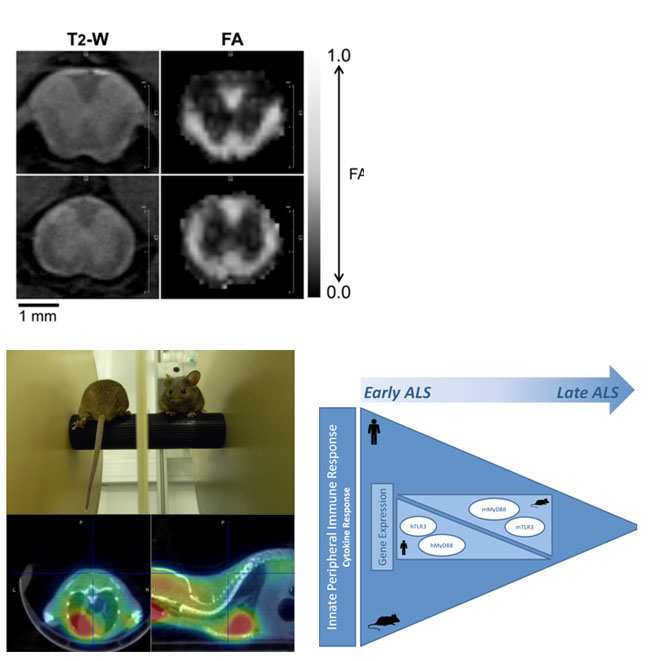
Figure 4: Unpublished results from a study by our group in which we measured the FA of SOD1G93A mice using Echo-planar imaging (EPI), a very fast acquisition data scheme that allowed us to increase the number of diffusion directions to 81. Reduced FA values vere found en early stages of disease progression, suggesting that DTI MRI could be used for early diagnosis and follow up of ALS in preclinical resarch. Below, CT-PET imaging in SOD1G93A mice and rotarod test (NIR Lab) together with one of our working model in clinical settings.
Clinical Collaborators and Industrial Partnerships
The CMN Lab has a joint research program in MS/ALS led by Dr. M. Garcia-Toscano, Scientific Director of Amarna Therapeutics (Holland) in Spain
The clinical partners inlude Dr. Guillermo Izquierdo (HU Virgen Macarena, Seville) and Dr. Celedonio Marquez-Infante (HU Virgen del Rocio-IBiS, Seville).
Transfer technology activity
2015 – P2015-31271
2015 – EP15382160.8 (with B. Gauthier’s Group at CABIMER)
2013 – P2013-31334
2012 – WO-2012-101309A1
2010 – WO-2010-037878A1
2009 – WO-2009-095516
Research Funding
Spanish Ministry of Science of Education
Spanish Ministry of Health (Instituto de Salud Carlos III)
Junta de Andalucía (Consejería de Economía, Innovacion, Ciencia y Empresa)
Junta de Andalucía (Consejería de Salud y Asuntos Sociales)
University of Seville
Fundación Salud 2000 Merck-Serono
Fundación Alicia Koplowitz
Bioiberica
Amarna Therapeutics
Treeway
Staff supported by
Miguel Servet Fellowship Program (Instituto de Salud Carlos III, Ministerio de Ciencia)
Juan de la Cierva Fellowship Program (Ministerio de Ciencia)
Federation of European Biochemical Societies (FEBS)
European Molecular Biology Organisation (EMBO)
Consejo Nacional de Ciencia y Tecnología (CONACYT). Gobierno de Mexico
JAE-CSIC Fellowship Program (Ministerio de Ciencia)
FPU Fellowship Program (Ministerio de Educación)
Plan Propio Universidad de Sevilla
Community Service
DP is associate editor for Journal of Nanoparticle Research (Springer)
DP is member of the editorial board of Nanomedicine (Elsevier)
Selected Publications
The chaperonin CCT inhibits assembly of α-synuclein amyloid fibrils by a specific, conformation-dependent interaction.
Sot B, Rubio-Muñoz A, Leal-Quintero A, Martinez-Sabando J, Marcilla M, Roodveldt C, Valpuesta JM
Scientific Rep. In press
Iron oxide nanoparticles as magnetic relaxation switching (MRSw) sensors: current applications in nanomedicine
D. Alcantara, S. Lopez ML Garcia-Martin, D. Pozo.
Nanomedicine. 2016 Jul;12(5):1253-62
The ‘Omics’ of Amyotrophic Lateral Sclerosis
Caballero-Hernandez D, Toscano MG, Cejudo-Guillen M, Garcia-Martin ML, Lopez S, Franco JM, Quintana FJ, Roodveldt C, Pozo D.
Trends Molecular Medicine 22: 53-67. 2016
Chaperome screening leads to identification of Grp94/Gp96 and FKBP4/52 as modulators of the α-synuclein-elicited immune response
Labrador-Garrido A, Cejudo-Guillén M, Daturpalli S, Leal MM, Klippstein R, De Genst EJ, Villadiego J, Toledo-Aral JJ, Dobson CM, Jackson SE, Pozo D, Roodveldt C.
FASEB J. 30: 000-000. 2016 (ahead october 6. 2015. DOI. 10.1096/fj.15-275131)
Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation
Chen SW, Drakulic S, Deas E, Ouberai M, Aprile FA, Arranz R, Ness S, Roodveldt C, Guilliams T, De-Genst EJ, Klenerman D, Wood NW, Knowles TP, Alfonso C, Rivas G, Abramov AY, Valpuesta JM, Dobson CM, Cremades N.
Proc Natl Acad Sci U S A. 112: 1994-2003. 2015
Betalactam antibiotics affect human dendritic cells maturation through MAPK/NF-kB systems. Role in allergic reactions to drugs
Lopez S, Gomez E, Torres MJ, Pozo D, Fernandez TD, Ariza A, Sanz ML, Blanca M, Mayorga C.
Toxicol Appl Pharmacol. 28 :289-99. 2015
Polysaccharide Colloids as Smart Vehicles in Cancer Therapy
Caro C, Pozo D.
Curr Pharm Des. 21: 4822-36. 2015
Synthesis of 1D-glyconanomaterials by a hybrid noncovalent-covalent functionalization of single wall carbon nanotubes: a study of their selective interactions with lectins and with live cells
Pernía Leal M, Assali M, Cid JJ, Valdivia V, Franco JM, Fernández I, Pozo D, Khiar N.
Nanoscale. 7: 19259-19272. 2015
Long-circulating PEGylated manganese ferrite nanoparticles for MRI-based molecular imaging.
M. Pernia-Leal, S. Rivera-Fernández, JM. Franco, D. Pozo, JM. de la Fuente, ML. García-Martín
Nanoscale. 7:2050-2059. 2015
Vasoactive intestinal peptide (VIP) nanoparticles for diagnostics and for controlled and targeted drug delivery
R. Klippstein, D. Pozo
Adv Protein Chem Struct Biol. 98: 145-168. 2015
Chaperoned amyloid proteins for immune manipulation: a-Synuclein/Hsp70 shifts immunity toward a modulatory phenotype
A. Labrador-Garrido, M. Cejudo-Guillén, R. Klippstein, EJ. De Genst, L. Tomas-Gallardo, MM. Leal, J. Villadiego, JJ. Toledo-Aral, CM. Dobson, D. Pozo, C. Roodveldt
Immunity, Inflammation and Disease 2: 226-238. 2014
Synuclein preconditioning of microglia strongly affects the response induced by Toll-like receptor (TLR) stimulation
C. Roodveldt, A. Labrador, E. Gonzalez-Rey, CC. Lachaud, T. Guilliams, R. Fernandez-Montesinos, A. Benitez-Rondan, M. Delgado, CM. Dobson, D. Pozo.
PLoS One 8(11):e79160. 2013
Hsp70 oligomerization is mediated by an interaction between the interdomain linker and the helical lid of the SBD
FA. Aprile, A. Dhulesia, F. Stengel, C. Roodveldt, JL. Benesch, P. Tortora P, CP. Robinson, X. Salvatella, CM. Dobson, N. Cremades
PLoS One 8(6):e67961. 2013
A rationally-designed six-residue swap generates comparability in the aggregation behaviour of a-synuclein and ß-synuclein
C. Roodveldt, A. Andersson, E. de Genst E, A. Labrador-Garrido, CM. Dobson, GG. Tartaglia, M. Vendruscolo
Biochemistry 51: 8771-8878. 2012
Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers
B. Mannini, R. Cascella, M. Zampagni, M. van Waarde-Verhagen, S. Meehan, C. Roodveldt, S. Campioni, M. Boninsegna, A. Penco, H. Kampinga, CM. Dobson, F. Chiti Proc. Natl. Acad. Sci. USA, 109: 12479-12484. 2012
Nanoporous silica microparticle interaction with toll-like receptor agonists in macrophages.
M. Cejudo-Guillén, ML. Ramiro-Gutiérrez, A. Labrador-Garrido, A. Díaz-Cuenca, Pozo D.
Acta Biomater 8: 4295-4303. 2012
Rational design and development of nanotechnology-based vasoactive intestinal peptide (VIP) applications in health research
R. Klippstein, S. Lopez-Enriquez, D. Pozo
In “Hot Topics in Cell Biology” ISBN 978-1-909287-00-6. Pp 242-252. 2012.
Biohealthcare Publishing. Oxford, United Kingdom.
Engineered nanoparticles for improved vasoactive intestinal peptide (VIP) biomedical applications
R. Klippstein, D. Pozo
Recent Patents on Nanomedicine 1. 55-59. 2011
Nanotechnology based manipulation of dendritic cells for enhanced immunotherapy strategies
R. Klippstein, D. Pozo
Nanomedicine 6. 523-529. 2010
Activity-dependent neuroprotective protein (ADNP) expression in the amyloid precursor protein/presenilin 1 mouse model of Alzheimer’s disease
I. R. Fernandez-Montesinos, M. Torres, D. Baglietto-Vargas, A. Gutierrez, Gozes, J. Vitorica, D. Pozo
J Mol Neuroscience 41, 114-20. 2010
R. Fernandez-Montesinos, PM. Castillo, R. klippstein, E. Gonzalez-Rey, JA. Mejias, AP. Zaderenko, D. Pozo
Chemical synthesis and characterization of silver-protected vasoactive intestinal peptide nanoparticles
Nanomedicine (Lond). 4, 919-930. 2009
D. Pozo, P. Anderson, E. Gonzalez-Rey
Induction of alloantigen-specific human T regulatory cells by vasoactive intestinal peptide
J. Immunology 183, 4346-4359. 2009
C. Roodveldt, C.W. Bertoncini, A. Andersson, S-T.D. Hsu, R. FernaÅLndez-Montesinos, D. Pozo, J.C. Christodoulou, C.M. Dobson
Chaperone proteostasis in Parkinson’s disease: molecular insights into the Hsp70/a-synuclein complex
EMBO Journal 28, 3758 – 3770. 2009
JL. Herrera, E. Gonzalez-Rey, FJ Quintana, R. Fernandez-Montesinos, R. Najmanovich, D. Pozo
Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in
macrophages
J Cell Mol Med 13, 3209-3217. 2009
I. Barba, R. Fernandez-Montesinos, D. GarciÅLa-Dorado, D. Pozo
Alzheimer Disease beyond the genomic era: nuclear magnetic resonance (NMR) spectroscopy based metabolomics
J Cell Mol Med 12, 1477-1485. 2008
P M. Castillo, JL Herrera, R. Fernandez-Montesinos, C. Caro, AP. Zaderenko, J.A. Mejias, D. Pozo
Tiopronin monolayer-protected silver nanoparticles modulates interleukin-6 secretion mediated by Toll-like
receptor ligands.
Nanomedicine (Lond) 3, 627-635. 2008
D. Pozo
Immune-based disorders: the challenges for translational immunology
J Cell Mol Med 1085-1086. 2008








