Cell death signalling
In the last fifteen years there has been exceptional progress in understanding the signalling involved in the killing of tumor cells by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). In addition, the initial observation that TRAIL specifically induces cell death in tumor cells without affecting untransformed cells has inspired clinical trials with therapeutic agents designed to activate the apoptosis-inducing TRAIL receptors. Despite these advances, many questions still remain unresolved regarding the mechanisms underlying the resistance of normal cells to TRAIL. Furthermore, despite early promising results, a number of tumor cells, like those of the breast, pancreas, melanoma and neuroblastoma are frequently refractory to TRAIL-induced apoptosis. However, a general consensus for resistance to TRAIL has not yet been identified.
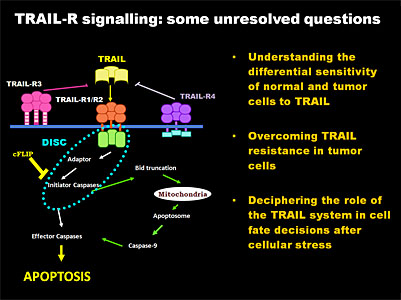
Our group has been investigating different aspects of apoptosis since 1989, with research grants from various sources and a number of publications in this field. Recent data from our group demonstrate that TRAIL induces cytoprotective autophagy in untransformed human breast epithelial cells. TRAIL-induced autophagy is mediated by the TAK1-dependent activation of the AMP-activated protein kinase (AMPK) that inhibits mammalian target of rapamycin complex 1 (mTORC1), a potent inhibitor of autophagy. These findings identify TAK1 as an activator of AMPK and cytoprotective autophagy and thereby as a regulator of cellular energy homeostasis and survival. The general goal of our current research is to elucidate the molecular mechanisms regulating TRAIL-induced apoptosis and autophagy in breast epithelial cells and to characterise the signalling pathways that generate resistance to TRAIL in tumor cells. In this respect, the main objectives of our research for the next five years are: a) to determine the relative contribution of cellular FLICE Inhibitory Protein (FLIP), autophagy, ER stress and cell cycle checkpoints to the resistance of cells to TRAIL and b) to investigate the regulation of TRAIL sensitivity during the epithelial-mesenchymal transition (EMT) in the progression of carcinomas.
Tumor microenvironment is characterized by glucose deprivation, acidosis, and severe hypoxia. These combined factors lead to the accumulation of misfolded proteins in the endoplasmic reticulum (ER), triggering the unfolded protein response (UPR) to facilitate tumor survival and growth. Besides the survival role of the UPR, different results suggest that activating the UPR in tumours by microenvironmental cues may modulate the expression of proteins of the extrinsic pathway of apoptosis, sensitizing these cells to TRAIL. Furthermore, our own results demonstrate that treatments that activate the UPR induce apoptosis, up-regulate the expression of TRAIL receptor 2 (DR5) and down-regulate the expression of anti-apoptotic proteins in different cell types. We are currently trying to identify the UPR branches that are involved in the regulation of these proteins.
Targeting mitotic exit has been recently proposed as a relevant therapeutic strategy to fight cancer with anti-mitotic drugs. In this respect, our recent results indicate that mitotic arrest sensitizes breast tumor cells to TRAIL by the proteasome-mediated degradation of the caspase inhibitor FLIP. Our goal is to understand the regulation of FLIP expression during mitosis and the role of cyclin-dependent kinase-1 (CDK1) in the control of FLIP levels and TRAIL sensitivity.
Regarding EMT and sensitivity of tumor cells to death receptor-mediated apoptosis, it has been reported that EMT confers resistance to cell death induced by TNF-alpha and CD95L. However, it remains unknown whether the transition to the mesenchymal phenotype leads to a reduced susceptibility to TRAIL. Because recombinant TRAIL and monoclonal agonistic antibodies against pro-apoptotic TRAIL receptors are currently undergoing clinical trials as anti-cancer agents, it is of pivotal importance to investigate the response of tumor cells to TRAIL during EMT. In this respect, we plan to investigate the role of FLIP in the regulation of TRAIL resistance in EMT and in particular, during tumor progression.
Funding:
– Frontera del Conocimiento. Junta de Andalucía (P20_00754)
– Ministerio de Ciencia, Innovación y Universidades (PGC2018-093960-B-I00)
– Centro de Investigación Biomédica en Red de Cáncer (CIBERONC) (CB16/12/00421)
– Red de Excelencia para el estudio de la Autofagia (BFU2015-71869-REDT)
– Unión Europea (FEDER)
Selected publications:
C. Ruiz-Ruiz and A. López-Rivas. p53-mediated up-regulation of CD95 is not involved in genotoxic drug-induced apoptosis of human breast tumor cells. Cell Death and Differentiation (1999) 6, 271-280
Ruiz-Ruiz, C. Muñoz-Pinedo and A. López-Rivas. Interferon-gamma treatment elevates caspase-8 expression and sensitises human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res. (2000) 60, 5673-5680
Sarker, C. Ruiz-Ruiz and A. López-Rivas. Activation of protein kinase C inhibits TRAIL-induced caspases activation, mitochondrial events and apoptosis in a human leukemic T cell line. Cell Death and Differentiation (2001) 8: 172-181
Muñoz-Pinedo, C. Ruiz-Ruiz, C. Ruiz de Almodovar, C. Palacios and A. López-Rivas. Inhibition of glucose metabolism sensitises tumor cells to death receptor-triggered apoptosis through enhancement of DISC formation and apical procaspase-8 processing. J. Biol. Chem. (2003) 278: 12759-12768
Ruiz de Almodovar, C. Ruiz-Ruiz, A. Rodríguez, G. Ortiz-Ferrón, J.M. Redondo and A. López-Rivas. TRAIL decoy receptor TRAIL-R3 is upregulated by p53 in breast tumor cells through a mechanism involving an intronic p53 binding site. J. Biol. Chem. (2004) 279: 4093-4101
Ruiz-Ruiz, C. Ruiz de Almodóvar, A. Rodríguez, G. Ortiz-Ferrón, J.M. Redondo and A. López-Rivas. The up-regulation of human caspase-8 by interferon-gamma in breast tumor cells requires the induction and action of the transcription factor IRF-1. J. Biol. Chem. (2004) 279:19712-19720
Palacios, R. Yerbes and A. López-Rivas. Flavopiridol induces cFLIP degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Research (2006) 66, 8858-8869
Gustavo Ortiz-Ferrón, Stephen W. Tait, Gema Robledo, Evert de Vries, Jannie Borst, and Abelardo López-Rivas. The mitogen-activated protein kinase pathway can inhibit TRAIL-induced apoptosis by prohibiting association of truncated Bid with mitochondria. Cell Death and Differentiation (2006) 13, 1857-1865
Griselda Herrero-Martín, Maria Høyer-Hansen, Celina García-García, Claudia Fumarola, Lone Batholm, Thomas Farkas, Abelardo López-Rivas@ and Marja Jäättelä@. TAK1 activates AMPK and AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. The EMBO Journal (2009) 28, 677-685
Tania Sánchez-Pérez, Gustavo Ortiz-Ferrón and Abelardo López-Rivas “Mitotic arrest and JNK-induced proteasomal degradation of FLIP and Mcl-1 are key event in the sensitization of breast tumor cells to TRAIL by anti-microtubule agents” Cell Death and Differentiation (2010) 17:883-94.
Carmen Palacios, Ana Isabel López-Pérez and Abelardo López-Rivas. Down-regulation of RIP expression by 17-Dimethylaminoethylamino-17-Demethoxygeldanamycin promotes TRAIL-induced apoptosis in breast tumor cells. Cancer Letters (2010) 287, 207-215.
Rosario Yerbes, Carmen Palacios, Mauricio J. Reginato and Abelardo López-Rivas “Cellular FLIPL plays a survival role and regulates morphogenesis in breast epithelial cells” BBA Mol Cell Res (2011) 1813, 168-178.
Rosario Yerbes, Abelardo López-Rivas*, Mauricio J. Reginato and Carmen Palacios*. Control of FLIPL expression and TRAIL resistance by the extracellular signal-regulated kinase (ERK)1/2 pathway in breast epithelial cells. Cell Death and Differentiation (2012) 19: 1908-1916.
Rosa Martín-Pérez, Carmen Palacios*, Rosario Yerbes*, Ana Cano-González, Daniel Iglesias-Serret, Joan Gil, Mauricio J. Reginato and Abelardo López-Rivas. (*equal contribution) Activated HER2 licenses sensitivity to apoptosis upon endoplasmic reticulum stress through a PERK-dependent pathway. Cancer Research (2014) 74: 1766-1777
Tania Sánchez-Pérez, René H. Medema and Abelardo López-Rivas. Delaying mitotic exit down-regulates FLIP expression and strongly sensitizes tumor cells to TRAIL. Oncogene (2015) 34: 661-669
Katiuska González-Arzola, Irene Díaz-Moreno, Ana Cano-González, Antonio Díaz-Quintana, Adrián Velázquez-Campoy, Blas Moreno-Beltrán, Abelardo López-Rivas and Miguel Á. de la Rosa. Structural Basis for Inhibition of the Histone Chaperone Activity of SET/TAF-Ib by Cytochrome c. PNAS (2015) 112: 9908-9913
Ana Cano-González and Abelardo López-Rivas. Opposing roles of TGF-b and EGF in the regulation of TRAIL-induced apoptosis in human breast epithelial cells. BBA Mol Cell Res (2016) 1863: 2104-2114
Rosa Martín-Pérez, Rosario Yerbes, Rocío Mora-Molina, Ana Cano-González, Joaquín Arribas, Massimiliano Mazzone, Abelardo López-Rivas@ and Carmen Palacios@. Oncogenic p95Her2/611CTF primes human breast epithelial cells for metabolic stress-induced activation of TRAIL-R/Caspase-8-dependent apoptosis.Oncotarget (2017) 8:93688-93703
Cristina Muñoz-Pinedo and Abelardo López-Rivas. A role for caspase-8 and TRAIL-R2/DR5 in ER-stress induced apoptosis. Cell Death and Differentiation (2018) (doi: 10.1038/cdd.2017.155.)
Ana Cano-González, Marta Mauro-Lizcano, Daniel Iglesias-Serret, Joan Gil and Abelardo López-Rivas. Involvement of both caspase-8 and Noxa-activated pathways in ER stress-induced apoptosis in triple-negative breast tumor cells. Cell Death and Disease (2018) 9(2):134. doi: 10.1038/s41419-017-0164-7.
Marta Mauro-Lizcano and Abelardo López-Rivas. Glutamine metabolism regulates FLIP expression and sensitivity to TRAIL in triple-negative breast cancer cells. Cell Death and Disease (2018) 9(2):205. doi: 10.1038/s41419-018-0263-0.
Santiago Serrano-Sáenz, Carmen Palacios, Daniel Delgado-Bellido, Laura López-Jiménez, Angel Garcia-Diaz, Yolanda Soto-Serrano, J Ignacio Casal, Rubén A. Bartolomé, José Luis Fernández-Luna, Abelardo López-Rivas* and F. Javier Oliver* (*senior and corresponding author) PIM kinases mediate resistance of glioblastoma cells to TRAIL by a p62/SQSTM1-dependent mechanism Cell Death and Disease (2019) doi: 10.1038/s41419-018-1293-3
Nathalie Tisch *, Aida Freire-Valls *, Rosario Yerbes*, et al… Abelardo López-Rivas, Thomas Schmidt, Hellmut G. Augustin and Carmen Ruiz de Almodovar Caspase-8 modulates physiological and pathological angiogenesis during retina development (*equal contribution) The Journal of Clinical Investigation (2019) doi: 10.1172/JCI122767
Rocío Mora-Molina, Daniela Stöhr, Markus Rehm and Abelardo López-Rivas cFLIP down-regulation is an early event required for endoplasmic reticulum stress-induced apoptosis in tumor cells Cell Death and Disease (2022) doi: 10.1038/s41419-022-04574-6.







