Pancreas and Liver Development and Disease
 Defects in organogenesis or function of liver and pancreas lead to debilitating diseases, including diabetes and cirrhosis. Understanding the processes by which these organs form during development and how cells are regenerated upon injury in adult tissue is critical to further our insights into how disease affecting these organs and how they might be treated in a more efficient manner than currently possible. Indeed, there is an urgent need for generating liver hepatocytes and pancreatic endocrine islets to treat severe liver failure and diabetes by transplantation. To accomplish this goal, it is imperative to fully understand how these organs are formed within the embryo and how they function at adult stages.
Defects in organogenesis or function of liver and pancreas lead to debilitating diseases, including diabetes and cirrhosis. Understanding the processes by which these organs form during development and how cells are regenerated upon injury in adult tissue is critical to further our insights into how disease affecting these organs and how they might be treated in a more efficient manner than currently possible. Indeed, there is an urgent need for generating liver hepatocytes and pancreatic endocrine islets to treat severe liver failure and diabetes by transplantation. To accomplish this goal, it is imperative to fully understand how these organs are formed within the embryo and how they function at adult stages.
Research Lines:
1. Molecular mechanisms of embryonic pancreas formation and adult pancreatic function
2. Molecular basis for hepatic fibrosis induction and progression
1. Molecular mechanisms of embryonic pancreas formation and adult pancreatic function
The pancreas is an essential organ that serves two vital functions: it makes digestive enzymes that aid in digestion and produce hormones that control blood glucose levels. Dysfunction of this organ might be caused by failures in the genetic program controlling the organogenesis process. Interestingly, many of these pancreatic embryonic pathways are also active in adult pancreas during normal and pathological conditions (diabetes, pancreatitis or pancreas cancer). In our lab, we are interested to understand how transcriptional networks control both pancreas embryonic formation and adult pancreas function, with emphasis on beta cell insulin-producing cells. Recent human genetic studies have identified new the transcription factors GATA4 and GATA6 as new players in pancreas organogenesis. Thus, an association between GATA6 and GATA4 mutations and human congenital pancreas agenesis has recently been reported. Using conditional knockout mouse models, we have elucidated the mechanisms underlying pancreatic agenesis linked to GATA mutations in humans. We have found that GATA4 and GATA6 are required for the proliferation and differentiation of the pancreatic progenitor cells. GATA factors are also expressed in adult insulin-producing beta cells suggesting that they might also play a role in adult beta cell function and/or glucose homeostasis. Indeed, mutations in GATA6 or GATA4 have been associated with adult onset of diabetes in humans. Currently, we are investigating whether deficiency in GATA factors might contribute to develop diabetes in humans.

2. Molecular basis for hepatic fibrosis induction and progression
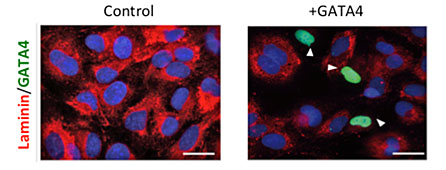
Liver fibrosis is a pathophysiological response to chronic injuries and requires the transformation of quiescent Hepatic Stellate Cells (HSCs) into an active and proliferative myofibroblast phenotype. Our lab is interested in understanding the molecular mechanisms of HSCs activation and in the identification of key players involved of this process. Our group has recently uncovered a critical role for GATA4 in HSCs function in both mice and human. Loss-of- function experiments in genetically modified mice have n that GATA4 is required to maintain the quiescent stage of HSCs. In activated human hepatic stellate cells, GATA4 overexpression is able to downregulate the expression of fibrotic genes. We are currently exploring the potential of GATA4 as an anti-fibrogenic agent. We have also determined that GATA4 expression in HSCs of human liver progressively decreases as the disease progress and it is dramatically reduced in patients with liver cirrhosis. Our lab is evaluating the expression of GATA4 in human liver as a potential biomarker of liver fibrosis stages.
Financial support:
-Instituto de Salud Carlos III
-Ministerio de Economía y Competitividad
-Junta de Andalucía (Consejería de Salud)
- Irene Delgado; Manuel Carrasco; Elena Cano; Rita Carmona; Rocío García-Carbonero; Luis Marín-Gómez; Bernat Soria; Francisco Martín; David Cano; Ramón Muñoz-Chápuli; Anabel Rojas*. GATA4 loss in the septum transversum promotes liver fibrosis. Hepatology. 59 – 6, pp. 2358 – 2370. 2014.
- David A. Cano; Bernat Soria; Francisco Martín; Anabel Rojas*. Transcriptional control of mammalian páncreas organogenesis. Cellular Molecular Life Science. 2013.
- Manuel Carrasco; Irene Delgado; Bernat Soria; Francisco Martín; Anabel Rojas*.GATA4 and GATA6 control mouse pancreas organogenesis. Journal of Clinical Investigation.122 – 10,pp. 3504 – 3515.2012.
- Anabel Rojas* ,William Schachterle-, Shaun-Mei Xu, Francisco Martín Bermudo, Brian Black. , Direct transcriptional regulation of GATA4 during early endoderm specification is controlled by FoxA2 binding to an intronic enhancer. Developmental Biology.346,pp. 346 – 355.2010.
- Anabel Rojas, Francisco Bedoya, Bernat Soria, Francisco Martín-Bermudo.Islet Cell Development. Advances in Experimental Medicine and Biology: Islets of Langerhams.654,pp. 59 – 75.2009.








